- Various studies have demonstrated that treatment with methyl prednisolone and chlorambucil could increase the chance of remission of idiopathic nephrotic syndrome (INS) of varied histology in patients who do not respond to the conventional treatment. This study was done to assess the safety and efficacy of methyl prednisolone and chlorambucil regimen in patients with various types of glomerulonephritides which were resistant to the usual conventional immunosuppressive drugs. Thirty nine patients were treated between June 1998 and December 2003 with Ponticelli regimen for six months. Twenty three patients (58.98%) were men and 16 (41.02%) were women. Mean age at the onset of NS was 23.59 ± 1.28 (range 10-51) years. Four patients (10.2%) had minimal change disease (MCD), six patients (15.4%) had membranoproliferative glomerulonephritis (MPGN), two (5.1%) had IgA nephropathy, and 18 patients (46.1%) had focal segmental glomerulosclerosis (FSGS). Eleven patients were excluded from the final analysis. Of the remaining 28 patients, mean baseline proteinuria was 3.31 ± 3.09 g/day. Mean baseline plasma albumin was 2.84 ± 1.002 g/dl and mean baseline serum creatinine was 0.87 ± 0.42 mg/dl. At the end of six months of treatment, mean proteinuria was 1.02 ± 0.85 g/day. Mean plasma albumin was 3.69 ± 0.78 g/day, and mean serum creatinine was 0.85 ± 0.26 mg/dl. Mean followup was 13.21 ± 7.7 times in 18.92 ± 12.58 months. At the end of six months of treatment, seven patients (25%) achieved complete remission (CR), 10 patients (35.71%) partial remission (PR), and 11 patients (39.3%) did not show any response to the therapy. Most of the patients in responder group had FSGS (64.70%), whereas in nonresponder group patients had MPGN and mesangioproliferative glomerulonephritis (MesPGN). Out of 13 FSGS cases five (38.46%) achieved CR, six (46.15%) PR, and only two (15.38%) failed to respond. The incidence of side effects was 39.3%. Responders had more side effects than nonresponders (47 vs 27.3%). Methyl prednisolone and chlorambucil therapy (Ponticelli regimen) is safe and efficacious in achieving remission in significant number of INS patients other than membranous nephropathy, without any serious side effect on short term followup. However, a longer followup is required to demonstrate the sustained efficacy and long-term side effect of this regimen.
-
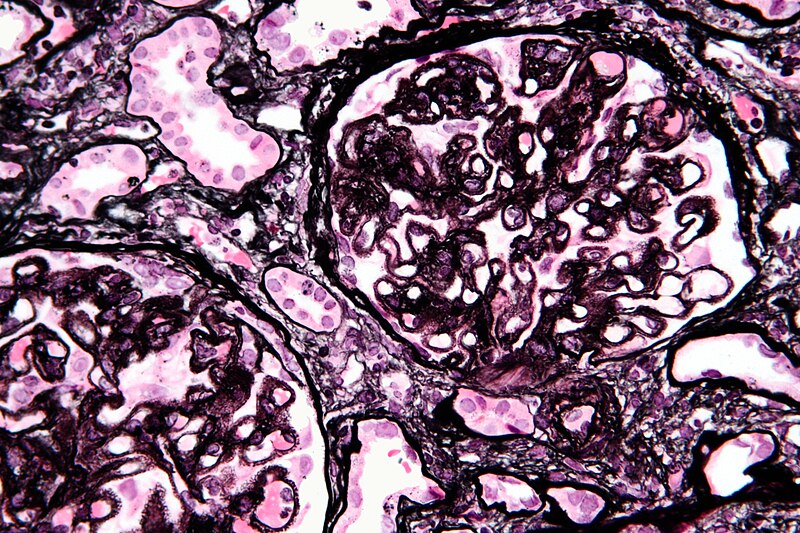
Membranous nephropathy
Primary/idiopathic
- 85% of MGN cases are classified as primary membranous glomerulonephritis -- that is to say, the cause of the disease is idiopathic (of unknown origin or cause). This can also be referred to as idiopathic membranous nephropathy. One study has identified antibodies to an M-type phospholipase A2 receptor in 70% (26 of 37) cases evaluated.[4]
Secondary
- autoimmu
- The remainder is secondary due to:
- infections (e.g., syphilis, malaria, hepatitis B)
- drugs (e.g., captopril, NSAIDs, gold, mercury, penicillamine, probenecid).
- inorganic salts
- tumors, frequently solid tumors of the lung and colon; hematological malignancies such as chronic lymphocytic leukemia are less common
Treatment
- Treatment of secondary membranous nephropathy is guided by the treatment of the original disease. For treatment of idiopathic membranous nephropathy, the treatment options include immunosuppressive drugs and non-specific anti-proteinuric measures.
Immunosuppressive therapy
- Corticosteroids: They have been tried with mixed results, with one study showing prevention of progression to renal failure without improvement in proteinuria.
- Chlorambucil
- Cyclosporine
- Tacrolimus
- Cyclophosphamide
- Mycophenolate mofeti
Natural history
- About a third of patients have spontaneous remission, another third progress to require dialysis and the last third continue to have proteinuria, without progression of renal failure.
Monday, 20 February 2012
Ponticelli regimen in idiopathic nephrotic syndrome
Friday, 17 February 2012
ABGs
Ph 7.69
PCo2 23
pO2 151
sO2 99%
HCO2 29
This ABGs belongs to a young male, dialysis-dependent for 1 year complained
Difficulty in breathing. He was just been dialyzed with an ultrafiltrate of 3 liters.
On examination, he was tachypenic. his blood pressure was 150/90.
PCo2 23
pO2 151
sO2 99%
HCO2 29
This ABGs belongs to a young male, dialysis-dependent for 1 year complained
Difficulty in breathing. He was just been dialyzed with an ultrafiltrate of 3 liters.
On examination, he was tachypenic. his blood pressure was 150/90.
The chest was clear on auscultation as well as on an x-ray chest
ABGs performed, shown above.
What does this blood gases show?
How will u manage?
Impression: Acute respiratory alkalosis with metabolic alkalosis
When ABGs were being drawn, the patient attended to pain, His rapid respiratory effort subsided.
In a couple of hours, he was alright and went home.
Hyperventilation (ie increased alveolar ventilation) is the mechanism responsible for the lowered arterial pCO2 in ALL cases of respiratory alkalosis.
Signs and Symptoms of Respiratory Alkalosis
Neurological
light-headedness
numbness and tingling
confusion
inability to concentrate
blurred vision
Cardiovascular
dysrhythmias
palpitations
diaphoresis
Miscellaneous
dry mouth
tetanic spasms of the arms and
legs
CLINICAL APPLICATION:
Treatment of respiratory alkalosis centers on resolving the underlying problem.
Patients presenting with respiratory alkalosis have dramatically increased work of
breathing and must be monitored closely for respiratory muscle fatigue. When the
respiratory muscles become exhausted, acute respiratory failure may ensue
Metabolic Alkalosis
Some causes of combined respiratory alkalosis with metabolic alkalois
Physiological: Pregnancy
In CCF when patient is tackypenic and he is being given diuretic.
In CLD: often seen when patient has vomiting too.
In Sepsis.
ABGs performed, shown above.
What does this blood gases show?
How will u manage?
Impression: Acute respiratory alkalosis with metabolic alkalosis
When ABGs were being drawn, the patient attended to pain, His rapid respiratory effort subsided.
In a couple of hours, he was alright and went home.
Hyperventilation (ie increased alveolar ventilation) is the mechanism responsible for the lowered arterial pCO2 in ALL cases of respiratory alkalosis.
Respiratory Alkalosis:
Respiratory alkalosis results from hyperventilation which is manifested by excess elimination of CO2 from the blood and a rise in the blood pH. Examples of specific causes are listed below:
| Central Causes (direct action via respiratory centre) |
|
| 2. Hypoxaemia (act via peripheral chemoreceptors) |
|
| 3. Pulmonary Causes (act via intrapulmonary receptors) |
|
| 4. Iatrogenic (act directly on ventilation) |
|
Neurological
light-headedness
numbness and tingling
confusion
inability to concentrate
blurred vision
Cardiovascular
dysrhythmias
palpitations
diaphoresis
Miscellaneous
dry mouth
tetanic spasms of the arms and
legs
CLINICAL APPLICATION:
Treatment of respiratory alkalosis centers on resolving the underlying problem.
Patients presenting with respiratory alkalosis have dramatically increased work of
breathing and must be monitored closely for respiratory muscle fatigue. When the
respiratory muscles become exhausted, acute respiratory failure may ensue
Metabolic Alkalosis
Metabolic alkalosis results from elevation of serum bicarbonate. Examples of specific causes:
- Volume contraction (vomiting, overdiuresis, ascites)
- Hypokalemia
- Alkali ingestion (bicarbonate)
- Excess gluco- or mineralocorticoids
- Bartter’s syndrome
Some causes of combined respiratory alkalosis with metabolic alkalois
Physiological: Pregnancy
In CCF when patient is tackypenic and he is being given diuretic.
In CLD: often seen when patient has vomiting too.
In Sepsis.
Tuesday, 14 February 2012
NEPHROTIC SYNDROM. cont
Algorithm for proteinurea

Secondary causes of nephrotic syndrome
• Diabetes mellitus
• Neoplasia
• Drugs
• gold
• antimicrobials
• NSAIDs
• penicillamine
• captopril
• tamoxifen
• lithium
• Infections
• HIV
• hepatitis B and C
• mycoplasma
• syphilis
• malaria
• schistosomiasis
• fi lariasis
• toxoplasmosis
• Systemic lupus erythematosus (SLE)
• Amyloid
• Miscellaneous
Complications of nephrotic syndrome
• Thromboembolism:
• DVT +/– pulmonary embolism;
• renal vein;
• arterial (rare).
• Infection:
• cellulitis;
• bacterial peritonitis (rare);
• bacterial infections, e.g. pneumonia;
• viral infections in the immunocompromised.
• Hyperlipidaemia;
• Malnutrition;
• Acute renal failure.

Secondary causes of nephrotic syndrome
• Diabetes mellitus
• Neoplasia
• Drugs
• gold
• antimicrobials
• NSAIDs
• penicillamine
• captopril
• tamoxifen
• lithium
• Infections
• HIV
• hepatitis B and C
• mycoplasma
• syphilis
• malaria
• schistosomiasis
• fi lariasis
• toxoplasmosis
• Systemic lupus erythematosus (SLE)
• Amyloid
• Miscellaneous
Complications of nephrotic syndrome
• Thromboembolism:
• DVT +/– pulmonary embolism;
• renal vein;
• arterial (rare).
• Infection:
• cellulitis;
• bacterial peritonitis (rare);
• bacterial infections, e.g. pneumonia;
• viral infections in the immunocompromised.
• Hyperlipidaemia;
• Malnutrition;
• Acute renal failure.
Monday, 13 February 2012
PROTEINUREA
Urinary protein excretion greater than 150 mg/day that persists beyond a single measurement should be evaluated. Proteinuria may be benign or suggestive of glomerular disease. Heavy proteinuria (>3 g/day), lipiduria, and edema are indicative of glomerular disease.There are three types of proteinuria: glomerular, tubular, and overflow proteinuria. Glomerular proteinuria accounts for virtually all cases of persistent proteinuria and is the only kind that is identified by urine dipstick. Qualitative tests for proteinuria include urine dipsticks and the sulfosalicylic acid test. These tests provide only rough estimates of the degree of proteinuria since they are influenced by the urine volume. The standard urine dipstick detects only albumin and is not sensitive enough to detect microalbuminuria. Sulfosalicylic acid (SSA) detects all proteins in the urine. Quantitative determination of the degree of proteinuria is provided by a 24-hour urine measurement. Such testing is cumbersome and an alternative, particularly for serial monitoring, is estimation of the total protein-to-creatinine ratio, which correlates with daily protein excretion. The optimal approach to the patient with proteinuria includes a thorough history, physical examination, and urinalysis. Transient and orthostatic proteinuria should be excluded. Persistent proteinuria warrants a thorough evaluation even when accompanied by a normal urine sediment. The evaluation should include measurement of serum creatinine and an ultrasound examination to rule out structural causes. Patients with persistent proteinuria should be referred to a nephrologist for decisions regarding further management including renal biopsy. A renal biopsy may be performed in the setting of nephrotic syndrome, increasing protein excretion, or an elevation in the plasma creatinine concentration. The renal prognosis of patients with glomerular proteinuria relates to the quantity of protein excreted. Non-nephrotic proteinuria (less than 3 g/day)
TYPES OF PROTEINURIA —
There are three basic types of proteinuria — glomerular, tubular, and overflow . Only glomerular proteinuria (ie, albuminuria) is identified on a urine dipstick. Almost all cases of persistent proteinuria are due to glomerular proteinuria.
Glomerular proteinuria —
Glomerular proteinuria is due to increased filtration of macromolecules (such as albumin) across the glomerular capillary wall. The proteinuria associated with diabetic nephropathy and other glomerular diseases, as well as more benign causes such as orthostatic or exercise-induced proteinuria fall into this category. Most patients with benign causes of isolated proteinuria excrete less than 1 to 2 g/day.
Tubular proteinuria — Low molecular weight proteins — such as ß2-microglobulin, immunoglobulin light chains, retinol-binding protein, and amino acids — have a molecular weight that is generally under 25,000 in comparison to the 69,000 molecular weight of albumin. These smaller proteins can be filtered across the glomerulus and are then almost completely reabsorbed in the proximal tubule. Interference with proximal tubular reabsorption, due to a variety of tubulointerstitial diseases or even some primary glomerular diseases, can lead to increased excretion of these smaller proteins .
Tubular proteinuria is often not diagnosed clinically since the dipstick for protein does not detect proteins other than albumin and the quantity excreted is relatively small. The increased excretion of immunoglobulin light chains (or Bence Jones proteins) in tubular proteinuria is mild, polyclonal (both kappa and lambda), and not injurious to the kidney. This is in contrast to the monoclonal and potentially nephrotoxic nature of the light chains in the overflow proteinuria seen in multiple myeloma.
Overflow proteinuria — Increased excretion of low molecular weight proteins can occur with marked overproduction of a particular protein, leading to increased glomerular filtration and excretion. This is almost always due to immunoglobulin light chains in multiple myeloma, but may also be due to lysozyme (in acute myelomonocytic leukemia), myoglobin (in rhabdomyolysis), or hemoglobin (in intravascular hemolysis) [6]. In these settings, the filtered load is increased to a level that exceeds the normal proximal reabsorptive capacity. Patients with myeloma kidney also may develop a component of tubular proteinuria, since the excreted light chains may be toxic to the tubules, leading to diminished reabsorption.
Some patients have mixed forms of proteinuria. As an example, glomerular diseases such as focal segmental glomerulosclerosis can be associated with proximal tubular injury, leading to tubular proteinuria. In addition, patients with multiple myeloma and Bence Jones proteinuria can also develop nephrotic syndrome due to AL (primary) amyloidosis.
Subscribe to:
Comments (Atom)